![]()
Signaling Networks and Tumor Modeling
Development of Multiscale Models of Tumor Progression
Tumor progression is the ultimate outcome of several time and space dependent interacting processes which entail the combined intracellular and extracellular events that govern cell survival, proliferation, and migration, as well as angiogenic, inflammatory, and immune responses. In this project we have developed a mathematical model that captures the complexity of tumor progression under biologically relevant conditions. Our agent-based model describes tumor growth and invasion as the outcome of proliferation and migration of individual cancer cells. The models consist of three interconnected components which describe the spatio-temporal distribution of nutrients and key signaling molecules (PDEs), the intracellular signaling pathway of each tumor cell (ODEs), and the state of each tumor cell (i.e., its phenotype, cellular mass, location). We use the model to explore hypothesis regarding the mechanisms underlying tumor progression. For instance, our simulation results suggest that the sensitivity of migrating tumor cells to chemoattractant gradients is an important determinant of the morphology and growth and invasion rate of the tumor.
Our overall goal is to develop a set of mathematical and computational tools for applications in cancer research. These applications include 1) integrating and organizing experimental data, 2) exploratory experiment in silico to get insights and proposed new experiments, 3) preliminary experiments to speed up and guide target identification studies, and 4) design of optimal drug scheduling policies.
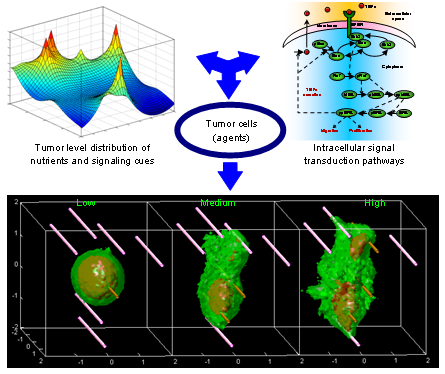
Figure Caption: Effects of chemotaxis on tumor progression
Analysis and Redesign of Signaling Networks
In this research, optimization based frameworks were developed for elucidating the input-output structure of signaling networks and for pinpointing targeted disruptions leading to the silencing of undesirable outputs in therapeutic interventions. The frameworks are demonstrated on a large-scale reconstruction of a signaling network composed of nine signaling pathways implicated in prostate cancer. The Min-Input framework is used to exhaustively identify all input-output connections implied by the signaling network structure. Results reveal that there exists two distinct types of outputs in the signaling network that either can be elicited by many different input combinations or are highly specific requiring dedicated inputs. The Min-Interference framework is next used to precisely pinpoint key disruptions that negate undesirable outputs while leaving unaffected necessary ones. In addition to identifying disruptions of terminal steps, we also identify complex disruption combinations in upstream pathways that indirectly negate the targeted output by propagating their action through the signaling cascades. By comparing the obtained disruption targets with lists of drug molecules we find that many of these targets can be acted upon by existing drug compounds while the remaining ones point at so far unexplored targets. Overall the proposed computational frameworks can help elucidate input/output relationships of signaling networks and help to guide the systematic design of interference strategies.

Figure Caption: Pictorial representation of the problems and solution strategies. The Min-Input framework (a) identifies the minimal sets of input signaling molecules that are capable of eliciting a particular cellular outcome. The Min-Interference framework (b) identifies the minimal combinations of disruptions to prevent an undesirable outcome while preserving a set of the desired outputs.


